Clinical Anatomy of the Vulva, Vagina, Lower Pelvis, and Perineum
Authors
INTRODUCTION
Clinical anatomy is concerned with the anatomic relationships between various structures of the living human organism. These relationships in the pelvis and their physiologic alterations along with the degree of independent function of each organ system are the concern of the gynecologist. Pelvic anatomy is complex and requires careful study and cadaveric dissection. A three-dimensional understanding of the interplay between bones, ligaments, pelvic organs, and muscles helps the gynecologist and pelvic surgeon identify and treat disorders of the pelvic floor.
In this chapter, the anatomy of the pelvic structures will be described from the clinician's point of view. The pelvis is divided into sections to facilitate understanding of its key components.
THE BONY PELVIS
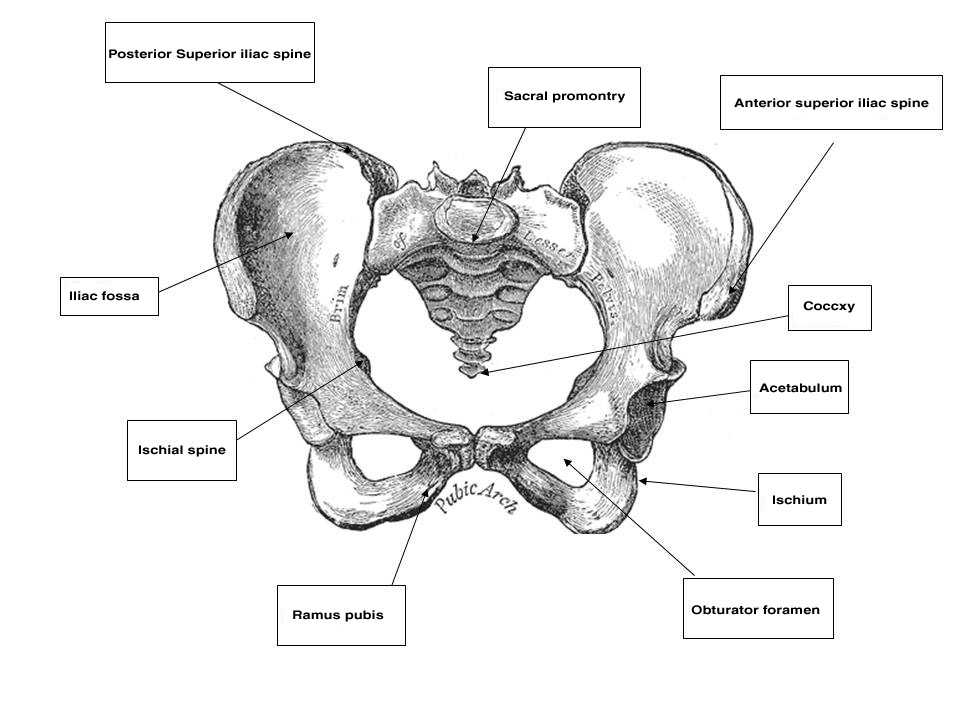
The pelvis is a basin-shaped structure that supports the spinal column and protects the abdominal and pelvic organs. It contains three pairs of hip bones: the ilium, ischium and pubis, as well as the sacrum and the coccyx. All three hip bones fuse together in the acetabulum. The pelvic bones serve as the origin and insertion points of the pelvic muscles and ligaments. Various parts of the bony pelvis can be used clinically as landmarks that are important to the obstetrician–gynecologist. Starting from back to front, these landmarks are the sacral promontory, which can be palpated on pelvic examination and is used for obstetric measurements of the birth canal as well as an anchoring point in apical suspension. The coccyx is an insertion point of the levator ani muscles. The ischial spine is used in obstetrics as a landmark for the station of the presenting part. It can also direct the examiner to the location of the pudendal neurovascular bundle, just posteriorly and laterally. In pelvic reconstructive surgery, it is the anatomical landmark that helps the surgeon identify the sacrospinous ligament that is running from the ischial spine to the sacrum. The anterior superior iliac spine (ASIS) and the posterior superior iliac spine (PSIS) are bony landmarks that assist the abdominal or laparoscopic surgeon in orientation and in placing the ports for laparoscopy. The inguinal ligament runs from the ASIS to the pubic tubercle. Finally, in the pubic bone there are the symphysis pubis, the pubic ramus which creates the obturator foramen with the ischium, the pectineal line in the back of this bone and the pubic tubercle. All these landmarks in the pubis are of great relevance for the pelvic reconstructive surgeon.
Fig. 1. Female bony pelvis (source, Gray's Anatomy of the Human Body, 1918)
GENERAL CONCEPTS OF PELVIC CONNECTIVE TISSUE HISTOLOGY
Before evaluating the specific structures concerned with the constituent and supporting tissues of the female pelvis, one must bear in mind the highly specialized purposes for which each component has been designed. The organs of the pelvis must be capable of individual distention to certain maximal limits in the course of performance of their natural duties, and yet they must be endowed with the ability to return to their original state. The components contain admixtures of various amounts of striated muscle, smooth muscle, collagen and elastic tissue. These act in concert, reinforcing one another but differing in certain specific functions and capability. Striated muscles play an important role in the support of the pelvic organs. These muscles include the pelvic diaphragm, which is made up of the levator ani and the coccygeus muscles and the lateral rotators of the thigh.1 They have a resting tone that can be modified both voluntarily and involuntarily as part of the normal function of the birth canal. The tendons and fascia of these muscles also play a major role in pelvic support. Smooth muscle fibers are in a constant state of activity and help to maintain tone. They are involuntarily mediated through the autonomic nervous system, spinal reflex arcs, and chemical or mechanical stimuli. Smooth muscles also show rhythmic contractility. Elastic tissue is made up of fibers constructed in irregular networks especially well developed in tissues usually subject to stress. These fibers respond to stress with stretching, but they also resist stretching by a natural tendency to return to their original state. Recent studies have demonstrated the role of elastin in pelvic support. Interference with the normal elastic fibers' homeostasis has been shown to cause pelvic organ prolapse in animals and humans.2, 3 Collagen fibers are also arranged in interlacing meshworks but, in contrast to elastic tissue fibers, are relatively not stretchable. They are, however, flexible, permitting movement in areas in which stretching is not intended, like a piece of string or the ropes on a swing. Type 3 collagen has been shown to be the primary collagen subtype in the vagina and supportive tissues. This type of collagen has relatively low tensile strength and provides flexibility to the tissue. Type 1 collagen, with its larger fiber size, provides higher tensile strength to the tissue. Recent studies have shown that further increase in type 3 collagen is associated with reduced tissue strength and prolapse.4
EXTERNAL GENITALIA AND VULVA
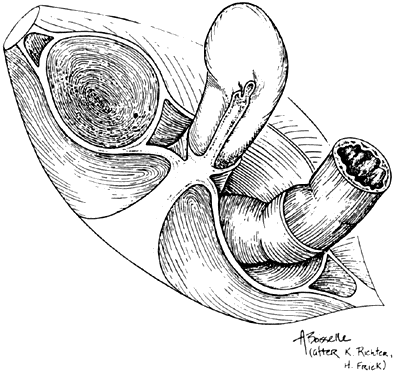
The female external genitalia, from front to back, consist of the mons pubis which extend into the two labia majora, the two labia minora that fuse at the level of the clitoris, the vestibule, the external urethral meatus, the hymen or its remnants, the ostia of the accessory glands (Bartholin’s glands, Skene’s glands and the vestibular glands) and the perineum that will be discussed later in this chapter. The mons pubis is a fat pad lying over the pubic bone and covered with hair. It contains skin appendages with sebaceous and sweat glands. The labia majora are bilateral folds of skin with underlying fat and appendages running from the mons pubis and merge in the perineum. The labia minora are found just medial to the labia majora. They contain no hair follicles and they fuse together anteriorly to form the prepuce and frenulum of the clitoris. The vestibule is the part of the vulva lying between the two labia minora laterally and extends medially to the hymenal sulcui. The Bartholin glands are located in the vestibule on either side. They produce mucus and participate in lubrication during sexual intercourse. Skene’s glands are a pair of paraurethral glands. In addition, there are minor vestibular glands located around the vestibule.
By drawing an imaginary line between the two ischial tuberosities, one can divide the pelvic outlet into two triangles. The urogenital triangle, with the pubic symphysis being its apex and anal triangle with its apex at the coccyx. The urogenital triangle is covered by the perineal membrane, a connective tissue layer that is lying between the pubic rami and is penetrated by the vagina, urethra and the rest of the external genitalia. The anal triangle consists of the anal canal, the internal and external anal sphincters, the ischiorectal fossa, and the median raphe. The muscles of the external genitalia include the superficial and deep transverse perineal muscles, the ischiocavernosus muscles that cover the crura of the clitoris, and the bulbocavernosus muscles.
Blood supply to the external genitalia is mainly from the pudendal artery and its branches to the urogenital and anal triangles. The lateral aspects of the external genitalia receive their blood supply from the external pudendal artery, a branch of the femoral artery. The mons pubis is supplied by the inferior epigastric artery, a branch of the external iliac artery. The innervation of the external genitalia is mainly by the pudendal nerve, which arises at S2–S4 levels and accompany the pudendal vessels. This nerve branches anteriorly to innervate the perineal membrane, external genitalia and the clitoris and posteriorly, the inferior rectal nerve supplies the ischiorectal fossa and the anal area. Two more nerves, the ilioinguinal (L1) and the genital branch of the genitofemoral (L1–L2), arising from the lumbar plexus, innervate the medial and lateral aspects of the vulvar skin respectively.
VAGINA
The vagina is a fibromuscular tube, the walls of which are normally in apposition in the relaxed state; it is H-shaped in its central portion, the side walls being suspended by their attachment to the paravaginal lateral connective tissue from which they receive their blood supply. It is lined by a stratified squamous epithelium thrown up into rugal folds, giving the epithelium accordion-like distensability without laceration. A dense, thin layer of elastic fibers is found immediately beneath the epithelium. Beneath this is a well-developed fibromuscular layer. The fibrous capsule external to this muscular coat is rich in elastic fibers and large venous plexuses. There are no glands in the vaginal lamina propria and vaginal lubrication is provided by transudate from the blood vessels as well as by secretions of the Bartholin’s and Skene’s gland.5 The vagina receives its blood supply from the vaginal arteries and their anastomoses with branches of the uterine, inferior vesical and internal pudendal arteries. The confluence of these anastomotic branches forms longitudinal azygous vaginal arteries in the midline of the anterior or posterior vaginal walls, or both, according to Smout and co-workers and Quinby.6, 7 A right and left vaginal artery, or occasionally two, arise (independently in most instances) from each internal iliac artery slightly cephalad and posterior to the origin of each uterine and inferior vesical artery. Occasionally, the vaginal artery arises as a division of a short common trunk with the uterine artery. This branching, however, occurs at the lateral extremity of each cardinal ligament and has great clinical significance. Arterial hemorrhage may thus follow laceration or surgical trauma to the vagina, especially in the vault of the vagina, even though the uterine artery has been securely ligated. Occasional postoperative arterial vaginal hemorrhage coexistent with intact ligation of the uterine artery may thus require separate isolation and ligation of the vaginal artery or, failing this, internal iliac ligation.
Normal Vaginal Axis and the Supports of the Vagina
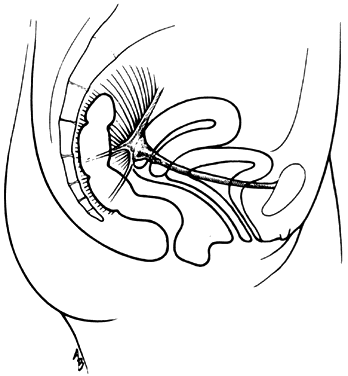
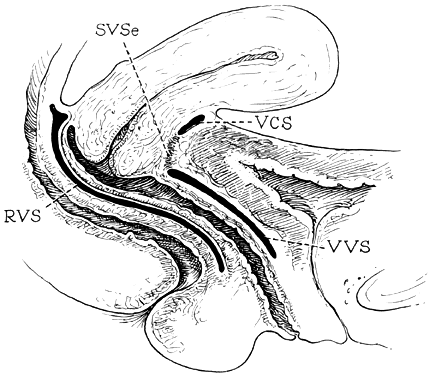
The vagina is attached laterally to the pelvic sidewalls by condensations of connective tissue and smooth muscle intimately adherent to the adventitia of the vaginal blood vessels. This tends to fix it in position from side to side, and the muscle elements supply a certain amount of tone, permitting it to adapt to changes in pressure. In the midline, the vagina is permitted freedom in distensability from both bladder and rectum by the relatively avascular vesicovaginal and rectovaginal spaces. The rectovaginal septum is fused with the posterior vaginal wall as the anterior lining of the rectovaginal space. The posterior vaginal wall is approximately 10 cm long. Since the cervix is incorporated in the anterior vaginal wall, the length of the anterior vaginal wall plus cervix approximates the length of the posterior wall. The connective tissue adventitia of the vagina is continuous with that of the cervix. The vagina is largest in its middle and upper third. The connective tissue lateral to the lower third is attached to fibers of the pubococcygeal muscle (fibers of Luschka) and to fibers fixing it to the perineal membrane. There are two arcus tendinei on each side of the pelvis. The arcus tendineus levator ani (ATLA) runs from the back of the pubis to the ischial spine (Fig. 2). Medial to this is the arcus tendineus fascia pelvis (ATFP) (Fig. 3). Although there is individual variation in the distance between these two condensations at their origin and lateral extent, they come together at the ischial spine. There is a connective tissue bundle running between the anterior vaginal sulcus and the arcus tendineus. The arcus tendineus is an important structure at the pelvic sidewalls. It is a firm anchoring point used in pelvic reconstructive surgery to support the vagina. Paravaginal repair as well as procedures using mesh kits for pelvic reconstruction aim to restore the normal arcus-to-arcus support of the pelvic floor.
In the living healthy female, the upper vaginal axis lies in an almost horizontal plane when the patient is in a standing position. Normal support of the vagina and the uterus is achieved mainly by the pelvic floor muscles with the assistance of the fibromuscular connective tissue of the vagina, also known as the “endopelvic fascia”. When the striated muscles of the pelvis are relaxed temporarily, as during micturition or defecation, or permanently, in cases of pelvic relaxation, the connective tissue is solely responsible for the pelvic support.5 The term “endopelvic fascia” was used to describe a sheet of fibroareolar tissue following the blood supply, as a retroperitoneal mesentery, to the visceral organs. This tissue divides the retroperitoneal space into avascular planes. Anteriorly, the “pubocervical fascia” attaches the uterine cervix and the vagina to the pelvic sidewalls. The posterior vaginal wall is attached to the pelvic sidewalls by the “rectovaginal fascia”. Nevertheless, this layer should not be termed “fascia”. Histologically, it is the vaginal muscularis, i.e. the subepithelial fibromuscular layer of the vagina.1, 5 For this reason, the previously called “endopelvic fascia” will be termed “vaginal muscularis”. The upper vagina lies on the rectum, which, in turn, lies on and parallel to the levator plate (Fig. 4). It is this almost horizontal position of the supporting levator plate that accounts for a similar axis to the vagina. The levator plate is formed by the fusion of the levator ani muscles posterior to the rectum, from just behind the levator hiatus to their coccygeal insertion. The rectum, vagina, and urethra pass through the levator hiatus. Although the cervix and upper vagina have considerable mobility, they are more or less anchored in position over the levator plate by the cardinal–uterosacral ligament complex. Vaginal support, as described by DeLancey in 1992,8 is achieved at three different levels: Level I – The uterosacral-cardinal ligament complex, supports the upper vagina and the uterine cervix. Level II – The paravaginal support of the vaginal sidewalls to the arcus tendineus fascia pelvis. Level III – The support of the distal part of the vagina to the perineum. Damage to the upper level of the suspensory system can give rise to inversion of the upper vagina, often with elongation of the cervix and cul-de-sac hernia; damage at the lower levels supporting system is more likely to be associated with eversion of the lower vagina, including cyctocele, rectocele and descent of the perineum. In most cases, there is no one site or degree of damage that must be repaired or restored; there are many, and they occur in various combinations at various times of life, from different etiologic factors, in varying degrees, and with varying degrees of symptomatology and disability.
PERINEAL MEMBRANE AND URETHRAL SUPPORT
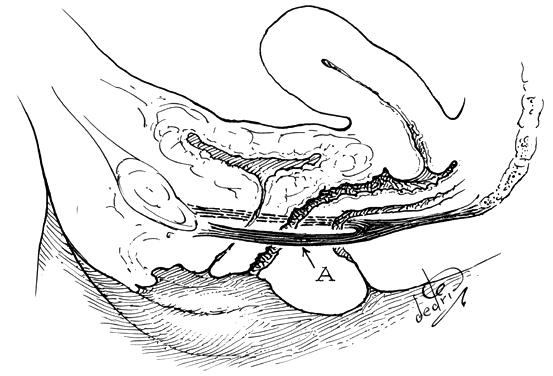
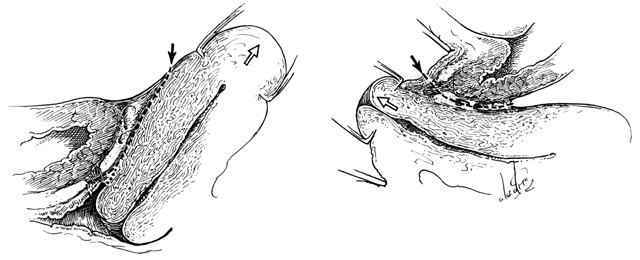
The perineal membrane, previously called the urogenital diaphragm, is a triangular structure of fibromuscular tissue that covers the anterior pelvic outlet. The change in terms reflects a recent different understanding of this structure. Rather then being a diaphragm (two layers of connective tissue with muscle between them), it appears to be a sheet of dense connective tissue.5The perineal membrane runs between the inner surfaces of the ischiopubic rami and is pierced in the midline by the urethra and vagina; by attachment to these structures, it assists in holding them in place. The posterior fibers of the perineal membrane are fixed to the perineal body. The superficial transverse muscle of the perineum, and the ischiocavernous and bulbocavernous muscles are located superficial to the perineal membrane deep within the soft vulvovaginal tissues; they appear to be considerably less important in urogenital support. The urethra is held in place by two systems. It is suspended by the perineal membrane and its attachment to the pubis, and supported by the connective tissue attachment between the anterior sulcus and the arcus tendineus (Fig. 5). Milley and Nichols9 studied the connective tissue supports of the urethra and confirmed the observations of Zacharin10 that the urethra is suspended from the pubic bone for most of its length by arched, bilaterally symmetric, anterior, posterior, and intermediate pubourethral ligaments. These studies further showed that the anterior and posterior ligaments are formed by reflections of the inferior and superior fascial layers of the perineal membrane. When studied by electron microscopy and neurohistochemistry, the smooth muscle bundles in this tissue are associated with numerous autonomic nerve fibers. The term ligament is therefore a misnomer, because these structures contain contractile elements under neural control.11
More recent studies by Oelrich, Gosling and others,12, 13 shed more light on the urethral support and sphincter mechanism. The urethral epithelium is continuous with the vaginal epithelium externally and with the bladder transitional epithelium internally. The epithelium is supported by a thin layer of lamina propria containing collagen and elastin fibers and small blood vessels. The urethral smooth muscle is mostly oriented longitudinally and obliquely and is under α-adrenergic and cholinergic control. This layer represents the intrinsic urethral sphincter mechanism. The striated periurethral muscles constitute the external urethral sphincter mechanism. The inner portion of this complex is made up of the sphincter urethrae – a circular muscle that surrounds the inner two thirds of the urethra, the compressor urethrae and the urethrovaginal sphincter (previously known together as the deep transverse perineus muscle). These three muscles are termed together the striate urogenital sphincter. The outer portion of this complex is composed of skeletal muscle fibers of the pelvic diaphragm. Contraction of the longitudinal smooth muscles as well as relaxation of the striated muscles of the sphincter complex allow micturition, whereas relaxation of these smooth muscles and contraction of the striated urogenital sphincter complex contribute to continence.5
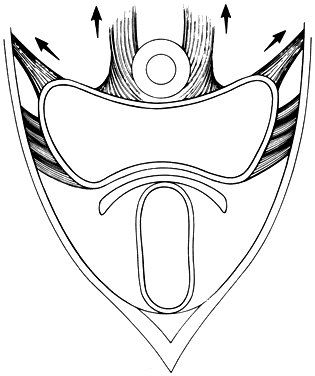 Fig. 5. The urethra is both suspended (central arrows show the attachments and pull of the pubourethral “ligament” portions of the perineal membrane) and supported (lateral arrows indicate the attachments of the vagina by intermediate connective tissue to the arcus tendineus). (Nichols DH, Randall CL: Vaginal Surgery, 3rd edn, p 21. Baltimore, Williams & Wilkins, 1989, with permission)
Fig. 5. The urethra is both suspended (central arrows show the attachments and pull of the pubourethral “ligament” portions of the perineal membrane) and supported (lateral arrows indicate the attachments of the vagina by intermediate connective tissue to the arcus tendineus). (Nichols DH, Randall CL: Vaginal Surgery, 3rd edn, p 21. Baltimore, Williams & Wilkins, 1989, with permission)
PERINEUM
The perineal body is a fibromuscular elastic structure found in the midline between the rectum and the vagina on a line between the ischial tuberosities; it contains much elastic tissue. It is subject to individual variation in tone, thickness, and composition. It is a point of convergence of various structures—the superficial and deep transverse perineus muscles, the bulbocavernous muscle, the external anal sphincter, some fibers of the levator ani (puborectalis and pubococcygeus muscles), the perineal membrane and the posterior vaginal muscularis. It is bounded anteriorly by the vagina and posteriorly by the rectum. The apex of the perineal body is continuous with the rectovaginal septum (the fascia of Denonvilliers), as shown in Fig. 6. When this attachment is avulsed, the weakness created favors the formation of a low or mid-vaginal rectocele. The apex of the perineal body must be reattached to the underside of the posterior vaginal wall and rectovaginal septum in order to rebuild the perineum. Recent studies have demonstrated the anatomy of the posterior compartment of the pelvis using axial magnetic resonance imaging and three-dimensional reconstruction from asymptomatic nulliparas. This compartment is bounded inferiorly by the perineal body, ventrally by the posterior vaginal wall, and dorsally by the levator ani muscles and coccyx. The support of the posterior compartment is achieved in the upper portion by the uterosacral ligaments, in the middle portion by direct contact with the lateral levator ani muscles and in the lower portion by fusion of the vagina and the perineal body.14
Blood Vessels of the Female Perineum
The blood supply to the perineal structures is derived from the branches of the internal pudendal artery, which arises from the anterior trunk of the internal iliac, leaves the pelvic cavity through the greater sciatic foramen, passes around the ischial spine, and enters the perineum by means of the lesser sciatic foramen. As it ascends along the pubic ramus, it pierces the perineal membrane, travels for a short distance within the membrane and gives off its terminal branches—the artery to the bulbocavernous muscle and the dorsal artery of the clitoris. The branches given off by this large vessel within the perineum include the inferior rectal (inferior hemorrhoidal) arteries given off as the vessel rises anterior to the ischial tuberosity. The inferior rectal arteries run across the ischiorectal fossa and are distributed to the anal sphincter and levator ani muscles. They are the chief sources of hemorrhage from all superficial wounds of the anus or ischiorectal fossa. These vessels have accompanying veins that empty into the pudendal veins. The superficial perineal or vulvar artery is given off anterior to the preceding branch. It is distributed to the vulva, with branches to the muscles, and is a source of arterial hemorrhage in wounds of the vulva. The transverse perineal artery is somewhat smaller, supplies the cutaneous surface of the perineum, and is therefore a source of hemorrhage from laceration of the perineal body. The fourth branch is the artery of the bulb, a vessel of considerable diameter but of short length. It sends branches to the bulbocavernous muscle. The terminal branches of the internal pudendal artery, the artery of the corpus cavernosum, and the dorsal artery of the clitoris are the supplying vessels of the erectile tissue of the clitoris. The veins of the perineum are valveless and have free anastomosis with the large intrapelvic venous plexuses. This situation permits alarming hemorrhage from obstetrical or surgical wounds of the vulva and vagina and the possibility of massive hematomas. Special properties of pelvic and perineal blood vessels include the following:
- Although there are many large venous networks within the pelvis that are capable of considerable venous distention, these veins are almost entirely without valves.
- Abundant smooth muscle fibers associated with adventitia of pelvic blood vessels probably account for at least part of the impressive quantity of smooth muscle found in the extraperitoneal connective tissue of the pelvis.
- The warmth and heat of tissues undergoing erection (clitoris, bulbocavernous muscle) demonstrate that most of the blood involved in the erectile process comes in fact from arteriolar dilation and that the venous congestion is probably a secondary phenomenon.
Neuroanatomy of the perineum
The pudendal nerve, arises from S2–S4, is responsible for the motor and sensory innervation of the perineum. This nerve exits the pelvis through the greater sciatic foramen, it hooks around the ischial spine and runs along the medial surface of the obturator internus muscle through the ischiorectal fossa in Alcock’s canal. It then travels posteriorly and medially to the ischial tuberosity and gives rise to its three branches: the inferior rectal (inferior hemorrhoidal), perineal and clitoral nerves.5 The mons pubis and anterior labia are supplied by the ilioinguinal and genitofemoral nerves. These nerves arising from the lumbar plexus and travel through the inguinal canal.
PELVIC DIAPHRAGM
The pelvic diaphragm is composed of the levator ani muscle and its superior and inferior fascial covering. This muscle functioned primarily as a tail-wagger in pronograde, four-legged animals. The assumption of the upright posture by man was accompanied by a loss of the tail as a functional appendage and the appropriation of this muscle for support of the pelvic viscera; in the erect posture the viscera have lost their previous inferior support by the pubis.15, 16 The levator ani muscle, acting in reciprocal concert with the muscles of the abdominal wall, has assumed much of the responsibility for support of both pelvic and abdominal contents and maintenance of equilibrium of intra-abdominal pressure.
Anatomy of the Levator Ani Muscle
The levator ani is composed of three general portions named according to the origin and insertion of each. These muscles are the pubococcygeus, puborectalis and iliococcygeus. The medial and anterior division is the pubococcygeus muscle, a somewhat V- or U-shaped sling that takes its origin from the back of the pubis on each side approximately 1.5 cm from the center of the symphysis. This portion is of the greatest importance to the gynecologist; the muscle is usually thicker along this medial margin than are the other two major divisions. The bellies of this 1- to 2-cm thick muscle sweep down and posteriorly along the sides of the urethra, the vagina, and then the rectum to insert into a fused median plate that runs from the tissue posterior to the rectum to the coccyx, the so-called levator plate. The most medial fibers form a loop behind the rectum. This U-shaped sling is called the puborectalis muscle. Some fascial and muscular fibers from the most anterior and medial portions intermingle with those at the sides of the urethra (pubourethral muscle), lower vagina (pubovaginal muscle), and perineal body, but some stronger bundles attach to the posterior lateral sides of the rectum (puborectalis muscle), and some fibers attach to the external anal sphincter. The last portion of the levator ani has been called the iliococcygeus muscle; it is somewhat thinner and flatter than the pubococcygeus and measures generally 0.5 to 1 cm in thickness. It takes its origin from the surface of the fascia of the obturator internus muscle along a line running from the posterior pubis to the ischial spine, the so-called white line or the arcus tendineus levator ani muscle. It is inserted into the lateral margin of the coccyx and lower sacrum. Another muscle, which is not a part of the levator ani, but makes up the posterior part of the pelvic floor is called the coccygeus muscle; it takes its origin from the ischial spine and inserts on the lateral margin of the coccyx and lower sacrum. It follows the course of the sacrospinous ligament; the muscle is in fact found on the superior aspect of this strong ligament.
The knowledge about the innervation of the levator ani muscles has been changed in the last decade by the work of Barber et al. (2002).17 By gross cadaveric dissections they were able to demonstrate that the levator ani muscle is not innervated by the pudendal nerve as was thought before, but rather by innervation that originates at the sacral nerve roots (S3–S5) and travels on the superior surface of the pelvic floor. This nerve was named the levator ani nerve.17
Physiology of the Levator Ani Muscle
The levator ani is a most unique and highly specialized voluntary muscle. Its various components are innervated by the pudendal nerve on each side, which supplies the external anal sphincter as well, hence these muscles tend to function in concert. Different constituent parts of the levator ani muscle perform different functions according to their anatomic location.18 These muscles are in a constant state of tone, which mediates the control of rectal continence. Neuromuscular pressure receptors within the striated muscular content of the levator ani are responsible for mediating this tone, and they apparently communicate with the central nervous system by way of the pudendal nerve on each side of the body. Congenital or acquired pathology of the pudendal nerve can alter the efficiency of its work, and thus influence the ability and efficiency of these neuromuscular receptors to maintain this responsive muscular tone. Acquired damage may result from stretching of the pelvic floor during childbirth or the chronic habit of excessive straining at stool. Similarly there may be congenital malformation affecting the pudendal nerves, most frequently from spina bifida.19 Damage to these neuromuscular receptors is not only slowly progressive over the years, but is virtually irreversible.20 Abnormalities in positions of the muscles can be corrected surgically, but abnormalities of innervation, either congenital or acquired, are refractory to surgical treatment. Prevention of neuropathy by skillful management of labor, and the elimination of constipation as well as pelvic floor exercises can help prevent this pathology.21 Although the levator ani, especially the iliococcygeal portion, is usually thought of as having a concave shape, pressure from fat within the ischiorectal fossa pushes the soft, yielding belly of the muscle upward and medially to a pouting convex shape when pressure is applied to the ischiorectal fat from below, as by sitting or reclining (Fig. 7).
Influence of the Pubococcygeus Muscle on the Mechanism of Voiding
The function of the pubococcygeal muscle in the normal voiding mechanism is described by Muellner.22 This concept emphasizes the importance of voluntary skeletal muscle in the mechanism of continence. Before urination begins, the diaphragm and the muscles of the abdominal wall contract, the intra-abdominal pressure rises, and the pubococcygei muscles relax. As the pubococcygei relax, the neck of the bladder moves downward. This downward movement activates or initiates contraction of the detrusor muscle. At the same time, the longitudinal fibers of the urethra, which are continuous with those of the detrusor, contract and shorten the urethra, thereby opening and widening the internal urethral orifice. Urine is then expelled from the bladder. At the conclusion of voiding, a contraction of the pubococcygeus raises the neck of the bladder, the detrusor and the urethral musculature relax, the urethra lengthens, the internal urethral orifice narrows and closes, and urination stops.
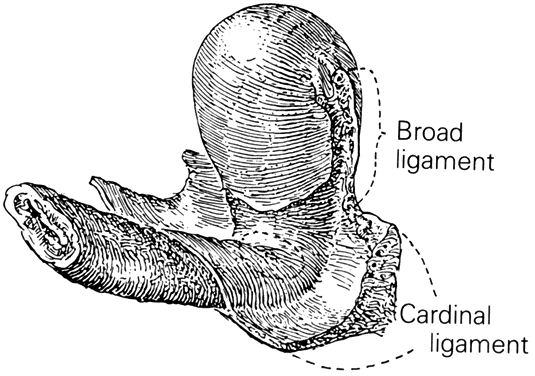
CARDINAL AND UTEROSACRAL LIGAMENTS
The cardinal and uterosacral ligaments are part of the suspensory apparatus (level I support) that serves to hold the uterus and upper vagina over the levator plate (Fig. 8). The blood vessels and lymphatics from the hypogastric plexus enter and leave the uterus and vagina along their lateral margins, as the vessels connect with their origin from the main internal iliac (hypogastric) vessels. Around these vessels are strong perivascular fibroareolar sheaths closely attached to their adventitia. Histologically, these ligaments consist principally of blood vessels (largely veins), nerves, lymphatic channels, and areolar connective tissue.
The uterosacral ligaments are attached to the posterolateral aspect of the cervix at the level of the internal os. There are fibrous attachments from the anterior third of the ligaments that course downward to attach to the lateral vaginal fornices. Near the cervix these ligaments are definite bands of peritoneum-covered tissue. As they course posteriorly, forming the superior boundary of the cul-de-sac of Douglas, they become thinned out with less definite peritoneal ridging. The posterior third of the ligament is fan-shaped and is composed of more delicate strands of tissue that attach to the presacral fascia opposite the lower portion of the sacroiliac articulation. There is much individual variation in the thickness and length of these ligaments and it is recognized that the ligaments do increase in prominence when tension or traction is applied to them. The uterosacral ligaments are, in fact, folds of peritoneum covering predominantly the pelvic parasympathetic fibers that pass anteriorly from the sacral plexus to the lateral aspects of the uterus. The uterosacral ligaments are of great importance to the pelvic reconstructive surgeon. Several procedures, both vaginal and abdominal have been described for the support of the vaginal apex or for prevention of future prolapse.23 The excellent and durable results of these procedures are a proof of the importance of these ligaments in apical support.
CONNECTIVE TISSUE PLANES AND SPACES OF THE PELVIS
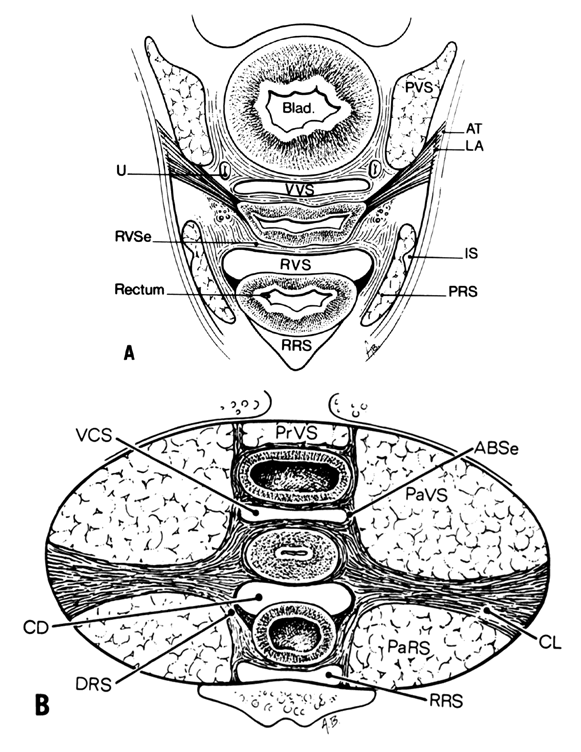
Much of the beauty of soft tissue pelvic architecture derives from the abilities of the organs of the three primary systems in this area—urinary, reproductive, and gastrointestinal—to function independently of one another. Each is capable of the limits of its normal range of function without permanent alteration of the anatomy or function of its neighbors. There are connective tissue spaces between these organs that permit this relatively independent function.24 These spaces are divided by connective tissue septa that not only afford mechanical support but also provide the physical routes of blood vessels, lymphatics, and nerve tissues to and from the pelvic organs. These structures are contained within the septa along reasonably constant routes and do not trespass on the connective tissue spaces. The anatomic ligaments form natural barriers to the spread of infection, cancer, and hematomas. The septa, on the other hand, through their blood vessels and lymphatics, form natural routes for the transmission of infection and malignancy arising from the pelvic organs. A detailed knowledge of the anatomy of these spaces and partitioning septa is essential to the understanding of their actual and potential functional importance in both health and disease. From accurate knowledge and experience, the surgeon can know not only where to find major vessels and so avoid unnecessary blood loss, but also how to avoid unnecessary surgical penetration of adjacent organs. To the oncologic surgeon, this anatomic knowledge helps to demarcate the likely limits and routes of direct spread of malignant disease and to determine the extent of necessary extirpation. To the surgeon concerned with pelvic reconstruction, the implications are obvious in the need to reestablish original relationships between the organs. The connective tissue capsules or adventitia of the bladder, birth canal, and rectum are attached to the pelvis, and at certain points to one another, by condensation of connective tissue that contain the principal blood vessels and lymphatics to and from these organs. Although these septa vary in strength and thickness from person to person, their relation and position are constant. Potential spaces exist between these septa, and the spaces are filled with fat and loose alveolar tissue but are essentially free of blood vessels and lymphatics (Figs. 9–11). These areas become actual spaces only by dissection, but this is easily accomplished bloodlessly and bluntly once access to the space has been gained by surgical penetration through a septum.
Safe extirpative or reconstructive surgery for benign pelvic disease requires identification, penetration, and invasion of the midline anterior and posterior spaces, but the oncologic surgeon requires penetration and dissection of the lateral spaces as well.
Vesicovaginal Space
The vesicovaginal space lies in the midline and is bounded anteriorly by the bladder adventitia, laterally by the bladder septa, or pillars, and posteriorly by the adventitia of the vagina. Superiorly it ends at the point of fusion between the adventitia of the bladder and vagina. This point of fusion is called the supravaginal septum or vesicocervical ligament.25 Inferiorly, the vesicovaginal space is limited by the fusion of the urethral and vaginal adventitia.
Supravaginal Septum
Anterior entry between the vagina and the peritoneal cavity is often through anatomic areas somewhat different, depending on whether the approach is from the vaginal or from the abdominal side. This structural difference may help explain why the surgeon who customarily operates by the abdominal route may experience unexpected difficulty in separating bladder from cervix when approaching a hysterectomy vaginally; similarly, the surgeon who is more comfortable with performing a hysterectomy through the vagina may wonder why unfamiliar difficulty may arise during the course of abdominal hysterectomy. This anatomic difference may be explained in Figure 12. A customary route of dissection is identified by the arrows. The vaginal operator may incise directly through the point of fusion between the bladder and the vagina, providing ready access to the anterior vesicouterine perineal fold. When this is not promptly evident, the physician may well have carried this dissection beneath the connective tissue capsule of the uterus, well above the anterior peritoneal reflection, and succeeded in peeling the peritoneum along with this uterine connective tissue capsule from the anterior surface of the uterus. The abdominal operator, on the other hand, will incise first directly into the anterior peritoneum, continuing the dissection beneath the connective tissue capsule of the uterus beneath or through the so-called supravaginal septum to the vagina. The former is the essence of the so-called endofascial hysterectomy. Recognizing these differences and becoming comfortable with both techniques will provide valuable surgical experience and enable one to find the anterior vesicouterine peritoneal fold when operating through the vagina, as well as finding the longitudinal muscle layer of the vagina more safely when operating for benign disease through a transabdominal approach.
Vesicocervical Space
The vesicocervical space is the continuation of the vesicovaginal space superiorly above the supravaginal septum. The posterior border becomes the connective tissue adventitia of the cervix, with which the adventitia of the vagina is continuous. The superior border is the peritoneum lining the vesicouterine peritoneal pouch. Cutting the supravaginal septum establishes communication between the vesicovaginal space and the vesicocervical space.
Ascending Bladder Septa
Although the ascending bladder septa are weak cephalad, they become the stronger bladder pillars (which contain efferent veins from the vesical plexus and ureter) by the addition of the lateral strong connective tissue portions of the cardinal ligament. Medially, they are loose in texture and contain fat and ureter. These septa contain the lateral inferior extensions of the bladder and connect it to the upper surface of the cardinal ligament, lateral to the cervix.
Prevesical Space of Retzius
the prevesical space of Retzius is in the form of a triangle extending from the umbilicus laterally to the lateral umbilical ligament (obliterated hypogastric artery). Anteriorly, the transversalis fascia extends from the umbilicus to the pubis; it extends inferiorly to the cardinal ligament and the supravaginal septum. It is separated from the paravesical spaces by the ascending bladder septa. The prevesical space thus includes the area between the pubis and the anterior vesical wall roofed by the fascia between the medial umbilical ligaments. The ascending bladder septum above the ureter contains many blood vessels including the inferior vesical artery and large veins of the vesical plexus. Below the ureter, however, blood vessels are scant and the tissues between bladder and vagina can be easily separated here without hemorrhage. The prevesical space of Retzius is the space developed during the Burch colposuspension procedure when the paraurethral tissue is attached to the iliopectineal (Cooper’s) ligament.
Paravesical Spaces
The paired paravesical spaces, right and left, are natural, fat-filled, preformed spaces that lie above the cardinal ligament and its prolongation (horizontal connective tissue ground bundle); they are bounded medially by the bladder pillars and laterally by the pelvic walls, the internal obturator muscle, and the levator ani. The roof is formed by the lateral umbilical ligament (vesicohypogastric fascia).
Descending Rectal Septa
The descending rectal septa run alongside the vagina from the undersurface of the cardinal ligament and its vaginal prolongation to the lateral surface of the rectum and thence to the sacrum.
Retrorectal Space
The retrorectal space lies in the midline between the sacrum and the adventitia of the rectum, between the posterior portion of the rectal pillars. This space communicates with the pararectal spaces above the uterosacral ligaments.
Pararectal Spaces
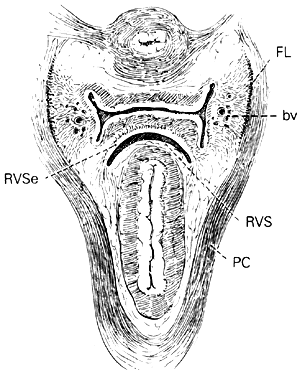
The paired pararectal spaces are only potential and are not preformed. They lie below the cardinal ligament and its vaginal prolongation. The medial border is formed by the rectal pillar, the lateral by the levator ani. The posterior portion extends backward above the ischial spine but under the cardinal ligament to the anterior surface of the lateral part of the sacrum. Behind the cardinal ligament the independent caudal portion of each side becomes continuous with the cranial portion of the opposite side. The upper rectum is surrounded by a single circular pararectal space. The boundaries of this space, formed by communication of two pararectal spaces and the retrorectal space, are formed laterally and below by the cranial surface of the levator, above and medially by the rectum, descending rectal septa, and the cardinal ligament. It is made L-shaped by the horizontal part below the cardinal ligament and the cranial and ascending portion behind the cardinal ligament. The cranial portion of the space is bounded anteriorly by the cardinal ligament and posteriorly by the lateral part of the sacrum. The sheaths of the great vessels of the pelvic wall form the lateral border; the pararectal space is bordered medially by the rectal septa and ureteric sheath. The inferior or horizontal division is bounded below by the levator ani, above by the cardinal ligament, and medially by the rectal septum. The two pararectal spaces communicate with each other posterior to the rectum, where there is no limiting membrane.
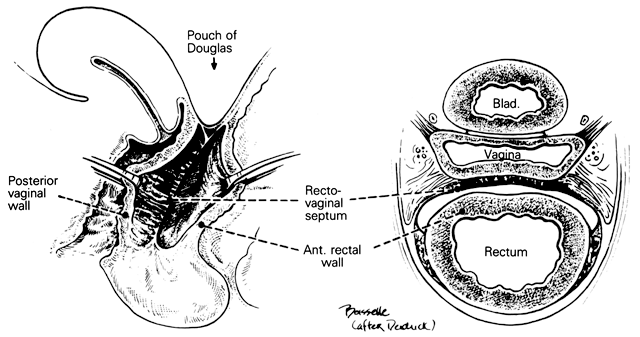
Rectovaginal Septum and Space
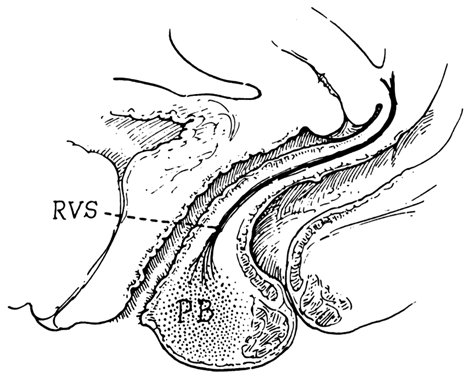
Centered in a relatively avascular rectovaginal space, the posterior vaginal wall and anterior rectal wall have functional independence of one another. This space permits the two organs to glide over one another with considerable mobility. The anterior wall of this space is formed by a specialized connective tissue layer of fused peritoneum, the rectovaginal septum.26, 27
As seen in sagittal section, this septum appears as a distinct, strong connective tissue layer between the vagina and rectum, oriented in a curved sagittal plane following the curvature of the pelvis. It is attached cranially to the caudal end of the peritoneum of the rectouterine pouch and extends inferiorly to the caudal attachment to the pelvic floor in the area of the perineal body. Anteriorly, the rectovaginal septum is always intimately attached to the posterior aspect of the vaginal connective tissue adventitia. Transversely, the septum curves posterolaterally, paralleling the course of the paracolpium (Fig. 13). Histologically, it consists of a fibromuscular elastic layer of dense collagen, abundant muscle, and coarse elastic fibers; the latter are best demonstrated with specialized orcein staining.
The rectovaginal space is in the midline between the rectovaginal septum, which is attached to the posterior vaginal wall, and the fat-covered rectal adventitia. The lateral walls are separated from the pararectal spaces by a descending rectal septum (rectouterine) on each side. The roof is the peritoneum and rectouterine peritoneal pouch (cul-de-sac of Douglas), and the inferior margin of this space is the perineal body.
CONCLUSION
Pelvic organ support and continence are maintained by a complex interplay of the bones, muscles and connective tissue structures of the pelvic floor. Understanding the anatomy of the female pelvis is fundamental for the obstetrician and gynecologist. More importantly, the pelvic reconstructive surgeon must have detailed knowledge of these structures in both normal and abnormal conditions in order to provide the best care for women with pelvic floor disorders.
REFERENCES
Kleeman SD Silva A: Gynecologic anatomy. In Sokol AI and Sokol ER, eds. General Gynecology: The Requisites in Obstetrics and Gynecology. Philadelphia, PA: Mosby/Elsevier, 2007, pp 73-97 |
|
Drewes PG, Yanagisawa H, Starcher B, et al: Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol 170(2): 578-89, 2007 |
|
Karam JA, Vazquez DV, Lin VK, Zimmern PE: Elastin expression and elastic fibre width in the anterior vaginal wall of postmenopausal women with and without prolapse. BJU Int 100: 346-50, 2007 |
|
Moalli PA, Shand SH, Zyczynski HM, et al: Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol 106(5 Pt 1): 953-63, 2005 |
|
Stepp KJ, Walters MD: Anatomy of the lower urinary tract, rectum and pelvic floor. In Walters MD and Karram MM, eds. Urogynecology and Reconstructive Pelvic Surgery. Philadelphia, PA: Mosby/Elsevier, 2007, pp 17-29 |
|
Smout CFV, Jacoby F, Lillie EW: Gynaecological and Obstetrical Anatomy. London: HK Lewis, 1969, pp 101–102 |
|
Quinby WC: The anatomy and blood vessels of the pelvis. In Meigs JV, ed. Surgical Treatment of Cancer of the Cervix. New York: Grune & Stratton, 1954, p 32 |
|
DeLancy JO: Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166: 1717, 1992 |
|
Milley PS, Nichols DH: The relationship between the pubourethral ligaments and the urogenital diaphragm in the human female. Anat Rec 170: 281, 1971 |
|
Zacharin RF: The suspensory mechanism of the female urethra. J Anat 97: 423, 1963 |
|
Wilson PD, Dixon JS, Brown ADG et al: Posterior pubourethral ligaments in normal and genuine stress incontinent women. J Urol 130: 802, 1982 |
|
Oelrich TM: The stiated urogenital sphincter muscle in the female. Anat Rec 205: 223, 1983 |
|
Gosling JA: The structure of the female lower urinary tract and pelvic floor. Urol Clin North Am 12: 207, 1985 |
|
Hsu Y, Lewicky-Gaupp C, DeLancey JO: Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol 198(6): 651.e1-7, 2008 |
|
Power RMH: Embryological development of the levator ani muscle. Am J Obstet Gynecol 55: 367, 1948 |
|
Thompson P: On the arrangements of the fasciae of the pelvis and their relationship to the levator ani. J Anat Physiol 35: 127, 1901 |
|
Barber MD, Bremer RE, Thor KB, et al: Innervation of the female levator ani muscles. Am J Obstet Gynecol 187(1): 64-71, 2002 |
|
Critchley HOD, Dixon JS, Gosling JA: Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int 35: 226, 1980 |
|
Swash MM, Henry M: Colpoproctology and the Pelvic Floor, 2nd edn. London: Butterworth-Heinemann, 1992 |
|
Parks AG: Anorectal incontinence. Proc R Soc Med 68: 21, 1975 |
|
Hay-Smith J, Bo K, Berghmans B, et al. WITHDRAWN: Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev (3): CD001407, 2008 |
|
Muellner SR: The anatomies of the female urethra. Obstet Gynecol 14: 429, 1959 |
|
Silva WA, Pauls RN, Segal JL, et al: Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 108(2): 244-5, 2006 |
|
von Peham H, Amreich J: Operative Gynecology, vol 1. LK Ferguson (trans). Philadelphia: JB Lippincott, 1934, pp 166–197 |
|
Shaw W: A study of the surgical anatomy of the vagina, with special reference to vaginal operations. Br Med J 1: 477, 1947 |
|
Milley PS, Nichols DH: A correlative investigation of the human rectovaginal septum. Anat Rec 163: 443, 1969 |
|
Nichols DG, Milley PS: Surgical significance of the retrovaginal septum. Am J Obstet Gynecol 108: 215, 1970 |